I find myself needing my phone the most when my battery is the lowest. It’s the Murphy’s Law of the connectivity era. And the more the device can do, the more juice you are likely to use. The panicked Arnold Schwarzenegger meme urging us to “get to the charger” sums up much of our pocket tech culture.
Yet, the future may see more juice than The Running Man himself. Toyota Research Institute of North America (TRINA) scientists have made a breakthrough centered on magnesium batteries. The innovation may provide a host of benefits for phone and car batteries.
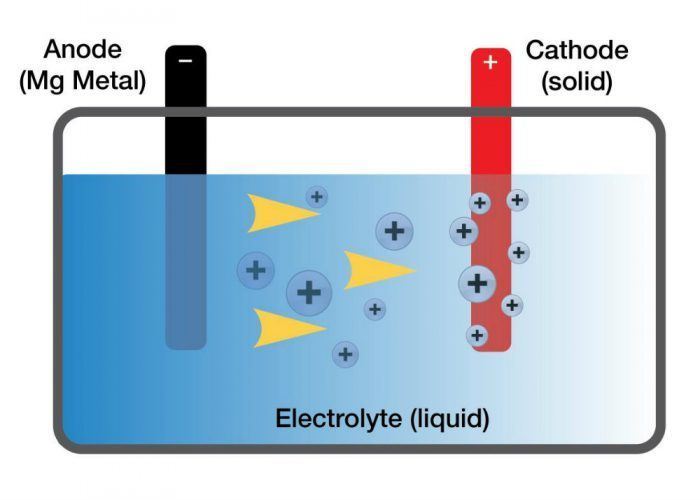
In theory, magnesium metal is safer and more energy-dense than current lithium batteries. Lithium metal, in its natural form, is unstable and can ignite when exposed to air. To keep the batteries we use daily safe, ions are taken from the lithium metal and embedded into graphite rods, which are then used in batteries.
The absence of actual metal limits the amount of battery power.
Magnesium, however, is more stable with the potential to store more energy. Research on this approach was limited because a magnesium-friendly electrolyte did not exist.
Until now.
Toyota principal scientist and chemical engineer Rana Mohtadi was researching hydrogen storage materials and their relevance to fuel cell technology. Because, you know, that’s what scientists and engineers do. While talking with her colleges, Mohtadi heard many of them discuss the difficulty of developing an electrolyte for an efficient magnesium battery.
I know, right . . . I’m thinking back to my water cooler conversations today too.
It didn’t take long for Mohtadi to conclude her hydrogen storage material might solve this. And with further experimentation, testing, and the help of fellow researchers, her theory proved correct.
“We were able to take a material that was only used in hydrogen storage and we made it practical and very competitive for magnesium battery chemistry,” Mohtadi said.
Toyota has long advocated a culture of collaboration and innovation. Such principals, when taken to heart, often lead to landmark discoveries like this.
“We try to put people from diverse backgrounds and diverse technologies together and allow them to collaborate,” said Energy Storage Group Manager Paul Fanson. “This is a great example of that working very successfully.”
I imagine longer lasting batteries would be immensely popular. Still, it could take up to 20 years before magnesium batteries reach the consumer market.
Toyota plans to further develop the technology but for now, one of the best discoveries, according to Fanson, is how their team can achieve great things.
“The results really speak to the strength in our group,” he said.
A paper detailing the discovery called An Efficient Halogen-Free Electrolyte for Use in Rechargeable Magnesium Batteries, was recently published in Angewandte Chemie International Edition (Vol. 54, Issue 27).
*Carl Anthony is Managing Editor of Automoblog and resides in Detroit, Michigan.
*Source: Toyota Motor North America



